NovaBone Announces Strategic Partnership with Summit Products Group to Distribute NovaForm® Wound Matrix in the U.S.
NovaBone Press Releases
Strategic collaboration brings NovaBone's innovative dental graft technology to European markets, debuting at IDS 2025 in Cologne.
"NovaBone Secures FDA Clearance for Expanded Indications of Putty in Interbody Fusion Applications"
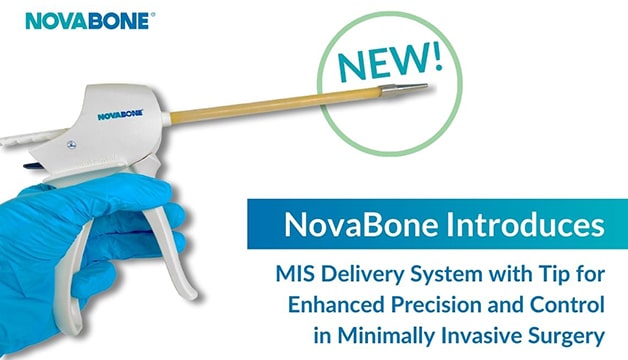
"Minimally invasive surgery is becoming increasingly popular due to its benefits for both patients and healthcare providers."
NovaBone enters an exciting phase of accessible digital services with its newly redesigned website.
NovaBone Products, leaders in synthetic biologics, is pleased to announce the company has made commercially available a new syringe for targeted delivery of its 45S5 bioactive glass putty device for the foot and ankle market. The newly launched syringe comes in both a 2.5cc and a 5cc formulation.
ALACHUA, FL June 27, 2023 - NovaBone Products, leaders in synthetic biologics is pleased to announce the company has received clearance from the U.S. Food and Drug Administration (FDA) for its proprietary Wound Matrix product – first of its kind collagen + 45S5 bioactive glass device for the wound care market.
The organization welcomes the New Vice President of Research and Development as they continue building momentum and improving the company’s capabilities.