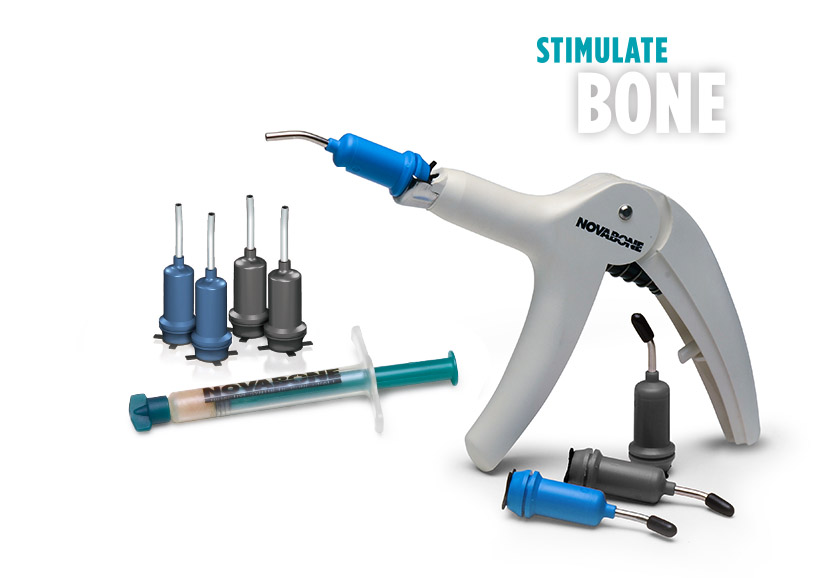
NovaBone Dental Putty®
Description
The next generation Calcium-Phosphosilicate bone substitute engineered for enhanced handling and predictable results. NovaBone® Dental Putty is ready to use and extremely user-friendly. It is pre-mixed, cohesive, moldable, and adaptable. NovaBone® Dental Putty is stable at room temperature, does not require refrigeration, has a 4-year shelf-life, and appears partially opaque on radiographs.
Clinical Indications
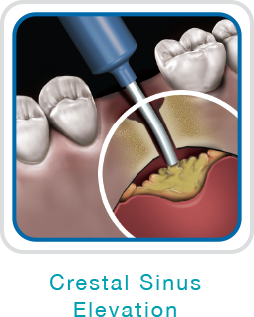
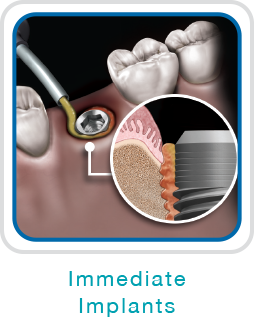
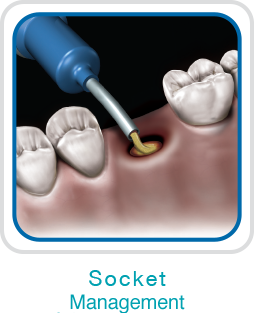
NovaBone Dental Putty is cleared by the US FDA for use in periodontal/infrabony defects, ridge augmentation (sinusotomy, osteotomy, cystectomy), extraction sites (ridge maintenance/augmentation, implant preparation/placement), sinus lifts, cystic cavities, and cranio-facial augmentation. It is particularly convenient for use in crestal and lateral cinus elevation, immediate implants, partial extraction therapies, socket preservations, and periodontal defects.
Unique Formulation
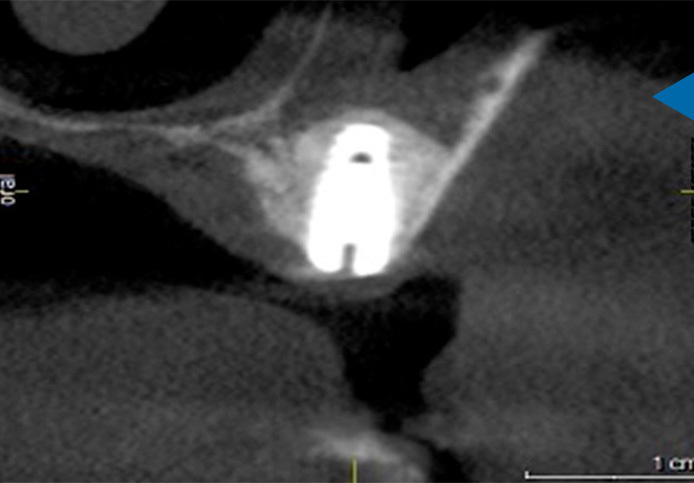
Grafting Simplified
Smart Science™
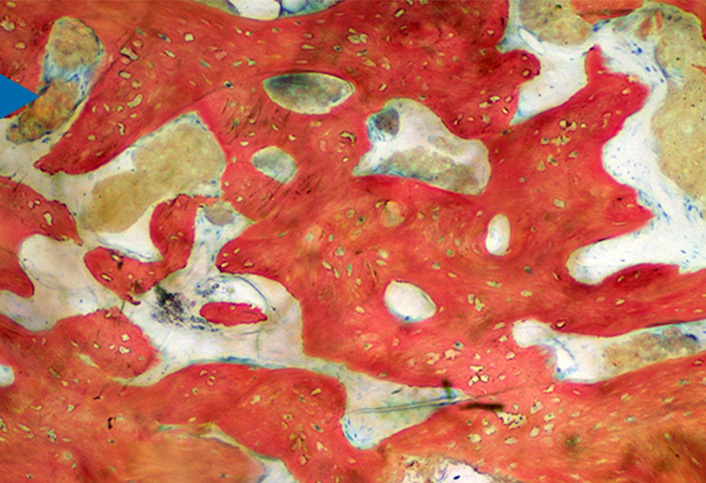
Unlike most synthetic grafts that are only osteoconductive, bioactive NovaBone® Dental Putty also has an "osteostimulative" effect. After implantation, surface reactions result in absorption of the graft material, a controlled release of Si, Ca, and P ions, and concurrent new bone formation. These surface reactions result in an osteostimulative affect, defined as the stimulation of osteoblast proliferation in vitro as evidenced by increased DNA content and elevated osteocalcin and alkaline phosphatase levels.
SIZES & AVAILABILITY
NovaBone Dental Putty is available in multiple delivery options: trays, pre-filled syringes and a unique cartridge delivery system. The diameter of the cannula is 2.8mm which is ideal for dispensing of the graft, especially in minimally invasive techniques such as gaps in immediate implant and crestal approach sinus lifts. Cartridges are available in various sizes and are used in conjunction with cartridge dispenser.
Products are available through a network of distributors world-wide, with a presence in over 40 countries. Not all dispensing formats are available in all countries.
Please check with your local distributor for sizes, configurations, and availability.
NovaBone Dental Putty Cartridge System
Description | USA Product Code |
|---|---|
Mini-Cartridges 4 x 0.25cc - Osteogenics | NA4640 |
Cartridges 2 x 0.5cc - Osteogenics | NA3620 |
Cartridges 6 x 0.5cc - Osteogenics | NA3660 |
Dental Putty Mini-Cart Dispenser - Osteogenics | NA4600 |
NovaBone Dental Putty Syringe Delivery
Description | USA Product Code |
|---|---|
0.5cc Syringe | NA1610 |
1.0cc Syringe | NA1611 |
2.0cc Syringe | NA1612 |
NovaBone Dental Putty Clam Shell
Description | USA Product Code |
|---|---|
1 x 0.5cc Shell | NA0610 |
6 x 0.5cc Shell | NA0660 |
Dental PerioGlas
Description | USA Product Code | International Product Code |
|---|---|---|
PerioGlas 2 x 0.5cc | NA0220 | EU0205 |
PerioGlas 6 x 0.5cc | NA0260 | EU0201 |
International Dental Putty
Description | International Product Code |
|---|---|
Dental Putty - 1 x 0.5cc Syringe | EU1610 |
Dental Putty - 2 x 0.5cc Syringe | EU1620 |
Dental Putty - 2 x 0.5cc Cartridge - Jumbo | EU3620 |
Dental Putty - 4 x 0.5cc Cartridge - Jumbo | EU3640 |
Dental Putty - 6 x 0.5cc Cartridge - Jumbo | EU3660 |
Dental Putty - 1 x 0.5cc Cartridge Jumbo | EU3699 |
Dental Putty - 4 x 0.25cc Cartridge - Jumbo | EU4640 |
Dental Putty Jumbo Cartridge Dispenser - NB | EU3600 |
PRODUCT LITERATURE
1. A simplified approach to the minimally invasive antral membrane elevation technique utilizing a viscoelastic medium for hydraulic sinus floor elevation. Kotsakis GA, Mazor Z. Oral Maxillofac Surg. 2015; 19(1): 97-101.
2. A clinical and radiographic case series of implants placed with the simplified minimally invasive antral membrane elevation technique in the posterior maxilla. Kher U, Ioannou AL, Kumar T, Siormpas K, Mitsias ME, Mazor Z, Kotsakis GA. J Craniomaxillofac Surg. Dec 2014; 42(8): 1942-7.
3. One-stage transaveolar vs. lateral maxillary sinus augmentation in severely resorbed sites using Calcium Phosphosilicate Putty: A proof of concept study. Kher U, Shanbhag S., Clin. Oral Impl. Res Oct 2014; 25(10).
4. Implants placed simultaneously with lateral-window sinus augmentation utilizing a calcium phosphosilicate putty alloplastic bone substitute for increased primary implant stability: A retrospective study. Udatta Kher, Ziv Mazor, Georgios A. Kotsakis, Panagiotis Stanitsas. Imp Dent 2014, 23(4):496-501.
5. Sinus Elevation with an alloplastic material and simultaneous implant placement: A 1-stage procedure in severely atrophic maxillae. Jodia K, Sadhwani B, Parmar BS, Anchlia S, Sadhwani SB., J Maxillofac. Oral Surg (July-Sept 2014) 13(3):271-280.
6. Minimally Invasive Crestal Approach Technique Utilizing a Cartridge Delivery System. Mazor Z, Ioannou A, Venkataraman N, Kotsakis G, Kher U., Implant Practice. Sept 2013; 6(4): 20-24.
7. Clinical and histologic comparison of two different composite grafts for sinus augmentation: a pilot clinical trial. Galindo-Moreno P, Avila G, Wang HL, et al., Clin Oral Implants Res. Aug 2008; 19(8): 755-9.



